About Medicamen Biotech Limited
Medicamen Biotech Limited ranks among the leading pharmaceutical companies with a focus
on delivering affordable, high-quality medicines worldwide. Medicamen envisions its future as
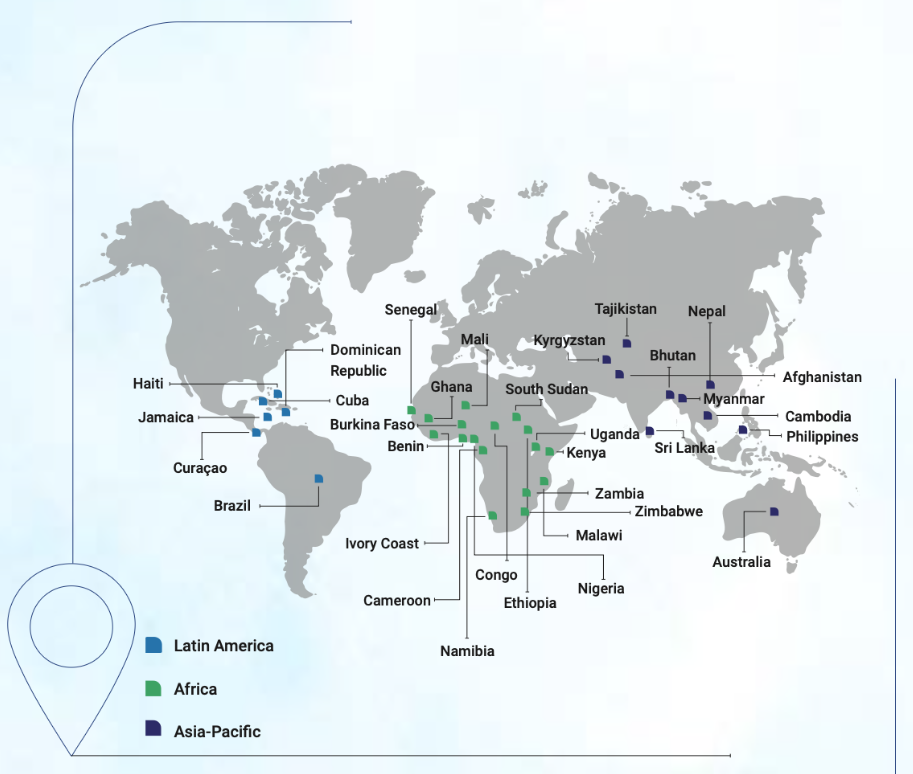
a global leader in this field. Medicamen operates WHO-GMP certified facilities in more than 35
countries, driving forward innovation and geographic expansion.
Established in December 1993, Medicamen has evolved into a fully integrated
pharmaceutical company, with expertise in research, Active Pharmaceutical Ingredient (API)
manufacturing and finished formulations. The Company's growth is supported by solid
infrastructure and innovation-led systems focused on quality and affordability, enabling
success in both regulated and semi-regulated markets.
Currently, the Company is developing general formulations intended for EU filings.
Medicamen is also actively expanding its oncology portfolio in Europe, Latin America, Asia-
Pacific and MENA through strategic partnerships, reinforcing its path to global leadership in
oncology generics.